In the highly regulated pharmaceutical industry, staying prepared for regulatory inspections is crucial to maintaining compliance, avoiding penalties, and safeguarding your reputation.
Mock-Inspection Audits act as a safety net, helping organizations identify gaps before actual inspections by authorities such as WHO, USFDA, or EU GMP. At Qualifyo, we bring decades of global expertise to simulate real-time inspection scenarios, ensuring your readiness and peace of mind.
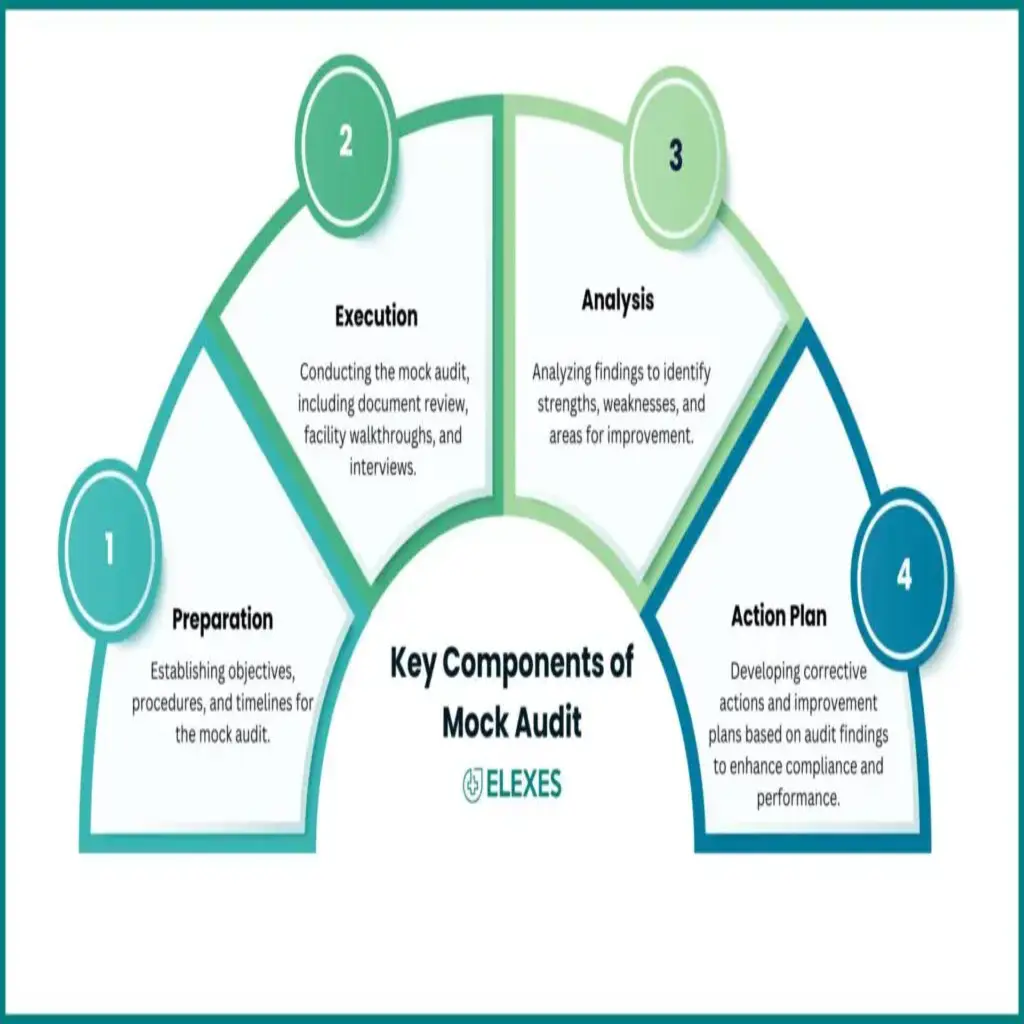
Comprehensive understanding of your compliance history, processes, and existing quality systems.
Simulated regulatory inspections with a focus on key areas like documentation, process validation, and data integrity
Identifying deviations, deficiencies, and risks compared to regulatory standards.
Providing detailed reports, risk categorization, and prioritized recommendations for corrective and preventive actions (CAPA).
Assistance with CAPA implementation, training, and follow-up audits to track progress.
detailing observations, deviations, and compliance gaps.
tailored to bridge gaps and align with global regulatory requirements.
Training sessions for your staff on addressing potential audit observations confidently.
inspection scenarios to prepare your team for actual audits
We’ve collaborated with leading regulatory experts worldwide and possess deep knowledge of WHO, PIC/s, EU GMP, and USFDA standards.
Every mock inspection is tailored to your facility’s unique processes and operational needs.
Trusted by top pharmaceutical organizations globally for robust, actionable results.
Beyond audits, we offer end-to-end assistance, including corrective measures, training, and follow-up evaluations.
Helping you prevent costly penalties, reduce operational risks, and safeguard your reputation.
Identify and mitigate risks before regulatory agencies identify them, protecting your organization from penalties and reputational harm.
Reduce the likelihood of non-compliance and unplanned production downtimes.
Avoid penalties, recalls, and reputational damage through proactive compliance.
Equip your team to confidently face audits with hands-on training and clear guidance.
Build trust with stakeholders by demonstrating a robust compliance culture.
Speak with our compliance experts to evaluate your readiness today.
Proactively identify gaps with our tailored audit services.

Speak to Our Experts at +91 9898573080
If you have a General enquiry, please drop me an email
Email: bd@qualifyo.com
Copyright © 2025 Qualifyo.